|
|
|
 |
|
|
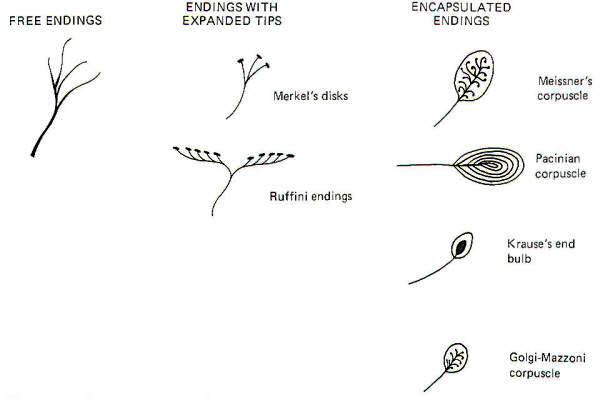
The central nervous system is kept continually informed of the ever-changing external and internal environment of the body by way of centrally directed signals which arise in its many and varied receptors. These receptors report on a wide variety of sensory modalities including changes in temperature, pressure, touch, sound, light, taste, smell, body and limb movements, and even blood pressure and chemistry. Scientists have recognized for almost 130 years that certain afferent nerve fibers of the peripheral nervous system are in contact with specialized non neural receptive structures which detect and transmit sensory information from the periphery to the CNS. The nonneural receptive structure together with its afferent nerve fiber is often called a receptor. Nature has evolved a variety of morphological structures which function as receptors. The earliest studies of sensation led to the idea that each morphological receptor type was responsible for the transduction of a particular modality of sensation. This early hypothesis has been modified in light of evidence that receptors respond to more than one type of stimuli.
An adequate stimulus is that form of stimulation to which a receptor has the lowest threshold. For example, a certain type of receptor will respond to a slight mechanical displacement by increasing the impulse firing rate in its afferent nerve fiber. The same receptor may also respond when subjected to extreme temperature changes. However, if it has a lower threshold for mechanical than for thermal changes, it is classified as a mechanoreceptor and not a thermoreceptor. Accordingly, receptors are often classified as follows:
Recognize that this classification does not mean that the adequate stimulus is the only stimulus to which a particular receptor will respond. It simply says that the receptor has the lowest threshold for (is most easily simulated by) the adequate stimulus.
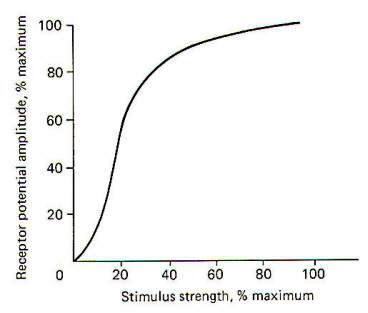
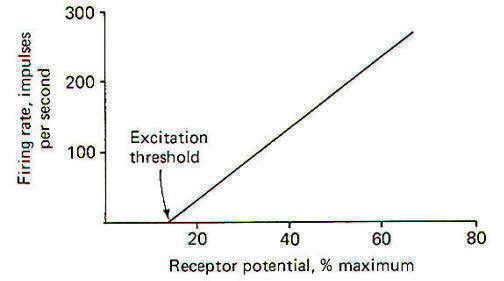
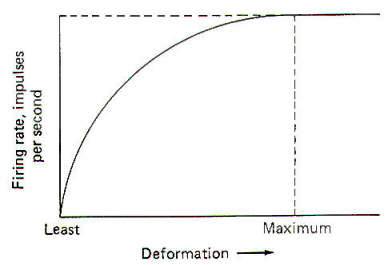
When a stimulus is applied to a receptor, it may or may not be strong enough to elicit impulse production in the afferent nerve fiber. The application of the stimulus causes the membrane of the receptor cell to depolarize, producing a receptor potential (RP). If the receptor potential reaches the excitation threshold of the nerve fiber membrane, the fiber will generate impulses. Further, as long as the receptor potential is maintained above the excitation threshold, impulses will continue to travel down the fiber away from the receptive element. A distinction can be made between receptors in which the receptive element is a specialized ending of the nerve fiber sharing a continuous membrane and receptors in which the receptive element is a separate structure not continuous with the membrane of the nerve fiber. In the former (one-element) receptor, the RP established in the receptive element produces impulses in the adjacent membrane by depolarizing this membrane with electrotonic currents. In the latter (twoelement) receptor the RP is generated in the separate receptive element, which in turn stimulates and produces impulses in the afferent nerve fiber. The mechanism by which a RP in the separate receptive element does this is not well understood. It may be that the close proximity of the two allows for a current spread between them or, as is suspected in some cases, a chemical transmitter may be released from the receptive element to the afferent nerve fiber. When not being stimulated, the membrane potential of the receptor is resting and polarized. However, when a stimulus is applied and its strength is steadily increased, the receptor membrane begins to depolarize and a RP is established (Fig-2).
It is thought that the receptor potential is produced by changes in the ionic current across the membrane of the receptive element. The depolarization phase of the receptor potential is very likely caused by the inward diffusion of Na+ ions. Repolarization is less well understood but is probably caused by ionic changes also. The receptor potential increases as a function of the stimulus strength and is therefore graded. However, it must be understood that Fig-2, which shows the relationship between the two, is based on a pacinian corpuscle from the cat mesentery and does not represent all types of receptors. Mathematical attempts have been made to predict the receptor potential from the strength of the stimulus but have thus far been inaccurate when applied to different types of receptors.
All three types of mechanoreceptors are found in hairy skin. While these three types are also found in glabrous (hairless) skin, there are significant differences between individual receptors. Position and Velocity Receptors Two types of position and velocity receptors are found in hairy skin. Type I receptors are the peripheral ends of type A beta fibers associated with Merkel's disks. They are stimulated by indentation of the skin and respond with an irregular discharge. They show a good velocity response but a smaller position response. Type II receptors are the peripheral ends of type A beta fibers which terminate in Ruffini corpuscles. They are stimulated by deformation of the skin and respond with a regular discharge. Unlike type I receptors, they have a good position response but a smaller velocity response. Both type I and type II receptors are slowly adapting and are thus able to give rise to conscious sensation associated with both instantaneous and prolonged skin displacement. Velocity Receptors Four types of velocity receptors are found in hairy skin. G2 hair receptors are the peripheral ends of type A beta fibers terminating around the base of guard hairs in the base of hair follicles. They respond to both slow and rapid movement of hairs and deflection of the skin. Field receptors are associated with type A beta fibers and their terminal morphology is unknown. They respond to indentation of the skin. D hair receptors are the terminal endings of type A delta fibers terminating around the base of both guard and down (fine) hair. They respond to both slow and rapid movements of these hairs as well as to skin deflection. C mechanoreceptors are rare, typically being associated with nonmyelinated type C fibers. Their terminal morphology is unknown and they respond only to slow displacement of the skin. Transient Receptors Two types of transient receptors are found in hairy skin. Pacinian corpuscle receptors are associated with the peripheral ends of certain type A alpha and beta fibers. They respond to mechanical "taps" and vibrations in the 50- to 500-Hz range. G 1 hair receptors are specialized processes at the base of hair follicles associated with large type A alpha fibers and they respond to high-velocity guard hair and skin displacement.
Like hairy skin, glabrous skin also contains position and velocity, velocity, and transient receptors. Nevertheless, there are some morphological differences between them such as the type of afferent nerve fiber which carries the signal and the nature of the receptive element itself. Position and Velocity Receptors The position and velocity receptors in glabrous skin are classified as slowly adapting (SA) receptors. It is likely that there is more than one type present. Nevertheless, SA receptors are associated with type A beta fibers and terminate in Ruffini-type corpuscles and possibly Merkel's disks. They respond to indentation of the skin. Velocity Receptors Velocity receptors in glabrous skin are classified as rapidly adapting (RA) receptors. RA receptors are associated with type A alpha fibers and possibly terminate in Meissner's corpuscles. Like SA receptors they respond to skin indentation. Transient Receptors The transient receptors in glabrous skin are also pacinian corpuscles. They have the same morphological and stimulating characteristics as those in glabrous skin.
Strict velocity receptors do not appear to be present in this group. However, transient receptors and several kinds of position and velocity receptors have been identified. Position and Velocity Receptors The position and velocity receptors in this group include the muscle spindles, Golgi tendon organs, and pressure receptors. Muscle spindles are associated with both group Ia and group II nerve fibers and respond both to change and rate of change in muscle length. Golgi tendon organs are the terminal endings of group Ib fibers and they respond to the tension developed in fascia and contracting or stretched muscle insofar as it applies tension to tendons. Pressure receptors respond to pressure on the belly of the muscle primarily, and to any distortion of the fascia surrounding the muscle. They are associated with certain group III fibers and their terminal morphology is unknown. Transient Receptors Transient receptors are again of the pacinian corpuscle type. They are associated with group II fibers and respond to both "taps" and vibrations in the 50- to 500-Hz range.
All three types of mechanoreceptors are represented in joints. However, their distribution is not uniform. Position and Velocity Receptors Position and velocity receptors are the most abundant type of mechanoreceptors found in joints. They fall into two categories. SA type 1 receptors are associated with myelinated fibers greater than 10 µm in diameter which terminate in Golgi-type organs. They are located in the joint ligaments and respond both to joint position and movement. SA type 2 receptors terminate in Ruffini-type endings and are associated with type A beta fibers. They respond to joint bending and discharge in the absence of movement to give position sense and during movement to give velocity sense. Velocity Receptors The velocity receptors signal phasic stimuli. Their terminal morphology is unknown but they are associated with type A alpha fibers and respond to joint movement, particularly of a bending and twisting nature. Transient Receptors This group represents the least common joint receptor responding to mechanical transients in joint movement. They signal "tap" stimuli and are associated with type A alpha fibers which terminate in paciniantype corpuscles. They discharge whenever the joint is moved regardless of the direction, and their response is brief.
The ear and the vestibular system make interesting use of mechanoreceptors. Organ of Corti hair cells respond to sound-induced movements of the basilar membrane of the inner ear. Special somatic afferent (SSA) fibers of cranial nerve VIII are stimulated when the hairs are bent. Vestibular system hair cells, located in the crista ampullaris and macula acustica of the vestibular apparatus, respond to angular movements, linear acceleration, and the position of the head in space. SSA fibers of cranial nerve VIII located at the base of the hair cells respond when the hairs are bent, pushed, or pulled.
A number of mechanoreceptors operate in the visceral organs and blood vessels. Carotid sinus and aortic baroreceptors, located in the walls of the carotid sinus and the aorta, respectively, respond to changes in blood pressure. Their terminal morphology is unknown, but general visceral afferent (GVA) fibers of cranial nerves IX (glossopharyngeal) and X (vagus), respectively, connect the receptive elements with the brainstem. Alveolar stretch receptors located in the walls of the pulmonary alveoli are the peripheral endings of GVA fibers of the vagus nerve. They respond to inflation and deflation of the lungs and their terminal morphology is unknown. Gastrointestinal (GI) stretch receptors are located throughout the walls of the GI tube from the pharynx to the rectum. They respond to stretch of the tube and subsequently conduct impulses to the CNS over GVA fibers of cranial nerves V (trigeminal), IX, and X as well as certain afferent fibers of the pelvic nerves. Urinary bladder stretch receptors are located in the walls of the detrusor muscle of the bladder. Their terminal receptive elements are associated with the GVA fibers of the pelvic nerve and they respond to filling of the bladder.
Receptors which respond primarily to injurious or painful stimulation are called nociceptors. Within this general category are four subgroups: mechanonociceptors, mechano-heat nociceptors, mechano-cold nociceptors, and poly modal nociceptors. Nociceptors are found in skin, muscles, joints, and the viscera.
Each of the four subgroups of nociceptors is represented in cutaneous tissue. While their terminal morphology is unknown, they are distinguished by their response patterns. Cutaneous mechanonociceptors are associated with type A delta fibers and respond to high shearing force. Cutaneous mechano-heat nociceptors respond to noxious levels of mechanical stimulation and heat in excess of 43°C. They are associated with certain myelinated type A delta fibers. On the other hand, cutaneous mechano-cold nociceptors are the terminal endings of certain nonmyelinated type C fibers. They are particularly adept at responding to noxious levels of mechanical stimulation and temperatures below 10°. Polymodal nociceptors respond to noxious levels of mechanical, heat, and chemical stimulation and represent the terminal endings of certain nonmyelinated type C fibers.
Two types of muscle nociceptors have been identified. Pressure nociceptors respond to strong pressure and excessive muscle stretch. Their terminal morphology is unknown and they are associated with myelinated group III fibers. Group IV nociceptors respond to strong pressure, temperature extremes, and anoxia. Their receptive elements are associated with nonmyelinated group IV fibers. Little is known about joint and visceral nociceptors. Joint nociceptors are the peripheral ends of certain type A delta fibers. They respond to joint overextension and their terminal structures are unidentified. Pain receptors in the viscera are probably not located in the parenchyma of the internal organs themselves, but are found instead in the peritoneal surfaces, pleural membranes, dura mater, and the walls of blood vessels. Chemoreceptors are defined as those receptors which respond most easily to chemical stimulation. External chemoreceptors include taste cells and olfactory cells, which give rise to the conscious sensations of taste and smell. Internal chemoreceptors respond to changes in circulating PCO2 PO2, and pH. They do not give rise to conscious sensation. Included in this category are the carotid body and aortic chemoreceptors and those chemoreceptors in the respiratory and vasomotor centers of the brainstem.
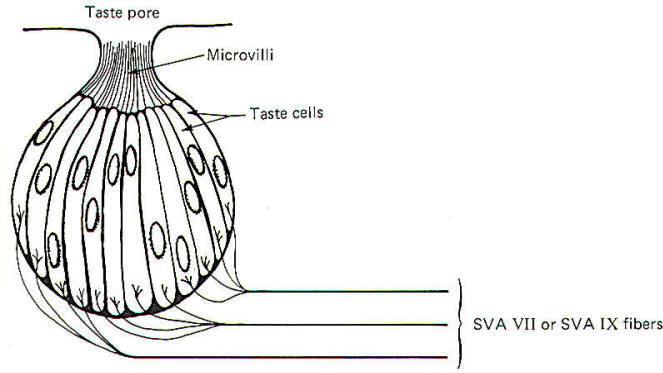
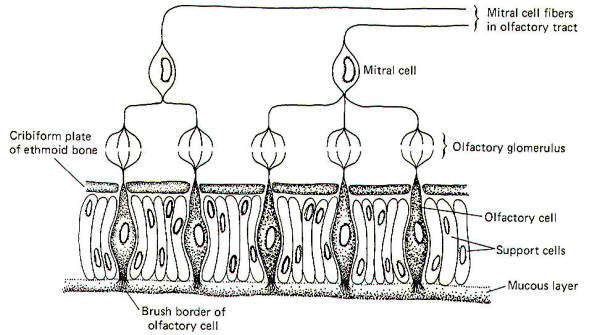
The taste cell is the chemically sensitive element for the sense of taste. Taste cells cluster together in small units called taste buds (Fig-9). The average taste bud contains 20 or so taste cells. Children have the greatest number of functional taste buds, and the number decreases with age so that the adult has about 10,000 functional buds. Each taste cell is typically columnar in shape and is characterized by numerous microvilli which project to a narrow opening at the top of the bud called a taste pore. The base of the taste cells are in close contact with the special visceral afferent (SVA) fibers of cranial nerves VII and IX.
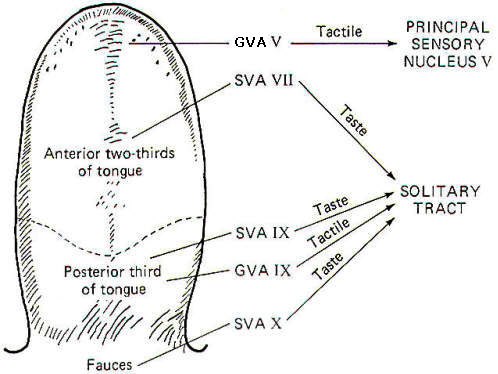
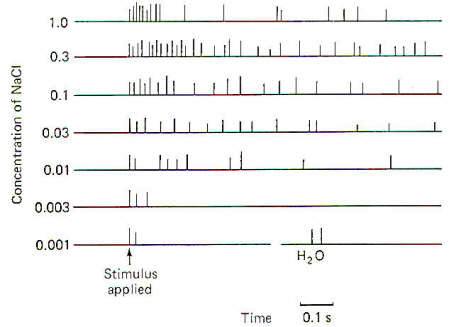
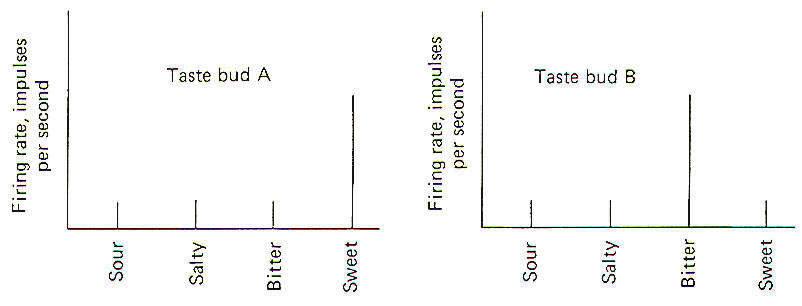
Papillae Location Taste buds are chiefly located in raised areas of the tongue known as papillae. In addition, taste buds are located on the epiglottis, the tonsilar pillars, and other areas of the fauces (passage from mouth to pharynx). Numerous small fungiform papillae are located over the anterior surface of the tongue. These papillae contain a moderate number of buds, perhaps as many as 100 per papilla. Much larger circumvallate papillae form a V on the back of the tongue and contain up to 250 taste buds each. Foliate papillae, located behind the circumvallate papillae, contain fewer buds. Afferent Innervation of the Tongue and Fauces Fig-10 illustrates that several afferent fibers conduct information from the tongue. Touch (not taste) sensation from the anterior two-thirds of the tongue is transmitted over GVA fibers of cranial nerve V to the principal sensory nucleus of V in the pons, while tactile sensation from the posterior one-third of the tongue is conducted over GVA fibers of cranial nerve IX to the solitary tract of the medulla oblongata. Taste sensation from the anterior two-thirds of the tongue is transmitted over SVA fibers of cranial nerve VII, while SVA IX fibers relay taste information from the posterior one-third. SVA X fibers conduct taste information from taste cells in the fauces. All of these afferent taste-conducting pathways terminate in the solitary tract. Four Basic Taste Modalities Four basic taste modalities are generally recognized. These are sweet, salty, sour, and bitter. Evidence suggests that all taste buds respond to some degree to all four stimuli. Nevertheless, buds on the tip of the tongue respond most strongly to sweet and salty stimuli, while chemicals giving rise to a sour sensation most effectively stimulate buds along the edge. Chemicals associated with the bitter sensation most effectively stimulate the base of the tongue. The adequate chemical stimuli for the four basic taste modalities fall into characteristic chemical groups. For instance, the chemicals which give rise to the sour sensation are usually acids. The lower the pH , the more the taste cells are stimulated. Sweet stimuli are usually organic molecules such as sugars, glycols, aldehydes, and others. Alkaloids like quinine, caffeine, and nicotine give rise to the bitter sensation, while ionizable salts give rise to the sensation we describe as salty. In order to stimulate the taste cells within a taste bud, the stimulating chemicals must dissolve in the saliva and then enter the taste pore. Here they stimulate the taste cells, which in turn stimulate the SVA endings of cranial nerves VII, IX, and X. Adaptation of Taste Cell Chemoreceptors When a taste stimulus is first applied to the tongue, the sensation is strong and then becomes weaker with time. The sourness becomes less sour, the sweetness becomes less sweet. etc. I n other words, the taste cells adapt to the stimulus. This subjective awareness of decreasing sensation is paralleled by a decrease in the firing rate of the SVA neurons (Fig-11). Taste Discrimination While taste buds respond to the four basic taste stimuli, they do so with different intensities. A particular taste bud may respond with a high-frequency discharge to a sweet stimulus but produce a lowfrequency discharge to salty, bitter, and sour stimuli. As stated previously, those responding primarily to bitter stimuli are concentrated at the base of the tongue, while those responding with the greatest discharge frequency to sweet and salty stimuli are concentrated at the tip. Sour receptors are located along the edge. Fig-12 illustrates the different sensitivities of two taste buds.
Taste bud A is a "sweet" bud. That is, when a sweet stimulus is applied, a much higher firing rate is initiated in the SVA fibers from its taste cells than when a bitter, salty, or sour stimulus is applied. Taste bud B, on the other hand, is a "bitter" bud because it responds with the greatest discharge to bitter stimuli. The brain probably interprets a given taste by analysis of the discharge ratios of the different kinds of taste buds stimulated. For example, if the firing rate from bud A is 10 times greater than from bud B when a chemical stimulus is applied, the stimulus was probably quite sweet. On the other hand, if the relative firing rates were reversed with bud B responding with a firing rate 10 times greater than bud A, the applied stimulus was probably quite bitter. Since the only message a neuron can carry is an impulse, all of which are quite similar, it follows that the only variable is the pattern of firing (i.e., the rate, grouping patterns, etc.). Consequently a possible partial explanation of how the conscious cortex evaluates a given taste stimulus is by analysis of the relative discharge patterns of the four basic kinds of taste buds from each part of the tongue and fauces. Such an integrated discharge pattern could supply the necessary information to the brain to enable it to accurately sense even the most subtle differences in taste. The chemically receptive element for the sense of smell is the olfactory cell. These cells, located in the olfactory mucosa of the nasal cavity, project their peripheral processes into a mucous layer which is exposed to the air in the nasal cavity. Their central processes penetrate the cribiform plate of the ethmoid bone to synapse with mitral cells in tufted olfactory glomeruli (Fig-13).
In addition to olfactory cells, the olfactory mucosa is also made up of support cells and mucus-secreting cells. The entire surface of the olfactory mucosa occupies a little more than 5 cm2.
The axons of mitral cells pass from the olfactory bulb centrally toward the brain as the olfactory tract. The tract then divides to form separate medial and lateral olfactory tracts. The lateral olfactory tract ultimately terminates in the periamygdaloid cortex of the temporal lobe. This pathway probably represents the conscious smell pathway. The medial olfactory tract may terminate in the septal nuclei, the contralateral amygdala, or the anterior continuation of the hippocampus. The body reflexly responds to both pleasant and unpleasant odors. The reflex responses are classified as viscerosomatic or viscerovisceral, depending on the nature of the response. Viscerosomatic reflexes include the reflex movements of the eyes, facial muscles, neck and the rest of the body in response to both pleasant and unpleasant odors. Viscerovisceral reflexes include salivary and gastric secretions in response to certain pleasant odors and vomiting in response to very obnoxious odors. Both the medial and lateral olfactory tracts contribute to the reflex pathways.
Unlike taste, no subjective classification of basic olfactory modalities has been agreed upon. However, for any odorant to be an effective stimuli it must be volatile. Water and lipid solubility are also desirable qualities. Volatility is necessary to allow the chemical to be adequately drawn into the nasal cavities, while water solubility is necessary since the odorant must penetrate the olfactory mucosa in order to reach the brush borders of the olfactory cells. There is even some evidence that the odorant must penetrate the brush border membrane in order to effectively stimulate the olfactory cell, in which case lipid solubility would be a desirable feature. In any event, the odorant establishes a receptor potential in the olfactory cell, which then gives rise to impulse production in the mitral cells of the olfactory bulb. The mechanism of olfactory cell stimulation of the mitral cells in unknown, but there is some evidence that a chemical transmitter may be involved. Olfactory Discrimination When an odorant of threshold concentration is presented to the olfactory epithelium, the subject is barely aware of its presence. If the concentration is increased, the sensation increases as well. Finally, the sensation reaches a maximum, and further increases in odorant concentration elicit no further increases in sensation. Allowing for individual differences, maximum sensation is usually reached with an odorant concentration 10 to 50 times greater than threshold. This does not allow much dynamic range. It is considerably less, for instance, than the range for vision (about 500,000 to 1). It would appear that the olfactory system is better designed for odor detection than for odor quantification. Further support for the idea that odor detection is perhaps the principal role of the olfactory system is the adaptation which occurs in the face of a sustained stimulus. The firing rate of olfactory tract neurons might decrease by as much as 50 percent within the first second or two following odorant application. This rapid decrease declines after the first second or two, but the signal is very weak after a minute or so. Electroolfactogram When an odorant is presented to the olfactory epithelium a monophasic action potential called the electroolfactogram (EOG) can be recorded. The amplitude of the EOG is a function of the odorant concentration, and in all probability represents the combined receptor potentials of many olfactory cells. Receptor potential recordings from individual olfactory cells has not yet been satisfactorily achieved.
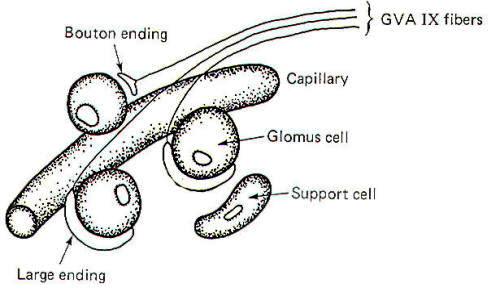
Internal chemoreceptors include the carotid body and aortic chemoreceptors and the chemically sensitive cells in the respiratory and vasomotor centers of the brainstem. The carotid body chemoreceptors have been subjected to more study than the others partly because of their relative accessibility. Recall that the internal chemoreceptors respond to changes in circulating PCO2, PO2, and pH but do not give rise to conscious sensation. Functional Arrangement of the Carotid Body Chemoreceptors The carotid bodies contain large glomus cells which make contact with the endings of the GVA fibers of the glossopharyngeal nerve. Two kinds of contacts are observed: small discrete bouton endings to single glomus cells and large endings in contact with several glomus cells (Fig-14).
Carotid Bodies Respond to Changes in PC02, P02, and pH The carotid bodies are particularly suitable for blood chemistry testing as about 20 ml per gram of carotid body tissue per minute is the flow rate of blood through the carotid bodies in the cat. This is among the highest tissue blood flow values found anywhere in the body. The carotid bodies are particularly sensitive to changes in the arterial oxygen concentration. When the P02 drops below the normal level of about 95 mmHg, the GVA fibers from the carotid bodies respond with an increase in their firing rates. It is not presently known whether the GVA endings themselves are directly stimulated by the oxygen drop or whether the glomus cells are the chemosensitive elements which then stimulate the bouton and large endings of the nerve fibers. To a lesser extent, the carotid bodies are also sensitive to changes in blood PC02, and pH. Increasing the PC02 above the normal value of 40 mmHg or decreasing the arterial pH below the normal value of 7.4 produces increased firing in the GVA IX fibers. Because of the close relationship between PC02 and pH it is difficult to tell which event is the actual stimulus. Again, it is not known whether the glomus cells of the endings of the afferent fibers themselves are initially stimulated. There is some evidence that chemical transmission is involved, however, and this would point to the likelihood that the glomus cells themselves are the actual receptive elements subsequently stimulating the afferent endings of the GVA fibers by chemical transmission. Evidence for Cholinergic Transmission If a chemical transmitter operates in the carotid body chemoreceptor system, it is probably acetylcholine. ACh is present in carotid body tissue. So are the enzymes necessary for its synthesis (cholineacetyltransferase) and degradation (acetylcholinesterase). In addition, carotid bodies in vitro are sensitive to extremely small amounts of ACh, and this sensitivity is enhanced by physostigmine (an anticholinesterase). In vitro studies also show that the response of the carotid bodies to natural stimulation is decreased by the administration of curare and atropine.
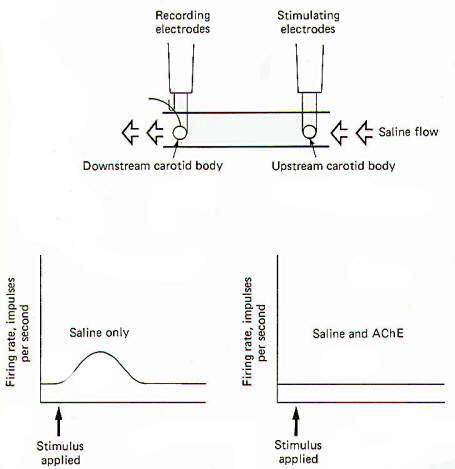
A technique pioneered by Otto Loewi has been used to illustrate the cholinergic nature of the carotid bodies. In Fig-15 two carotid bodies, each with its nervous innervation intact, are placed in a saline trough so that physiological saline can flow freely over both of them in a single direction. Stimulating electrodes are placed on the upstream preparation and recording electrodes are placed on the downstream preparation. When the two bodies are relatively close to each other (9 mm), stimulation of the upstream preparation produces, after an appropriate delay, increased firing in the nerve of the downstream preparation. The implication is that a chemical was released into the saline stream from the upstream preparation which diffused downstream, subsequently stimulating the downstream carotid body. Modification of this experiment indicates the chemical may be ACh. If the same procedure is run again with acetylcholinesterase (AChE) added to the saline flow, no response is observed.
The receptors of the peripheral nervous system will be classified (Table -1) according to the type of afferent nerve fiber which conducts its signals to the central nervous system. Receptors located within the brain (i.e., chemoreceptors of the hypothalamus and those in the respiratory and vasomotor centers of the brainstem) are not included in this classification system because they are not associated with peripheral terminations of spinal and cranial afferent nerve fibers.
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright [2007] [CNS Clinic-Jordan]. All rights reserved