|
|
|
 |
|
|
The ear is the organ and sound is the sensation of hearing. A neurophysiologist might define sound as a change in pressure propagated through an elastic medium (typically air) which is detected by the ear and sensed by the auditory cortex of the brain. An understanding of the characteristics of sound waves as well as the physics of mechanical transduction in the ear are important correlates to the study of hearing by the nervous apparatus. Those parameters of sound which are of particular interest in this regard are frequency, velocity, and amplitude.
The frequency f of a sound wave is equal to the number of oscillatory cycles it makes per unit time (typically per second). The human ear is sensitive to frequencies in the range between 10 and 20.000 Hz (cycles per second). Actually most people are sensitive to a narrower range between 50 and 10.000 Hz. Most speech is between 60 and 500 Hz and the ear is most sensitive to sounds in the 1200- to 4000- Hz range. The physical characteristics of the human hearing apparatus which favor this very sensitive range will be explained later.
The velocity c of a sound wave depends on the medium through which it travels. Typically the greater the density of the medium, the greater the sound velocity. For example, sound travels through air at 331 m/s, water at 1490 m/s, muscle at 1570 m/s, bone at 3360 m/s, and solids at 5000 m/s. The velocity of a sound wave is independent of its frequency. In other words, changing its frequency doesn't alter its velocity. If this were not the case, low notes from a musical chord might reach the ear at a different time than high notes from the cord, making the appreciation of music considerably less pleasant. The wavelength of a sound wave is equal to its velocity divided by its frequency and is thus expressed in distance per cycle. l =c/f
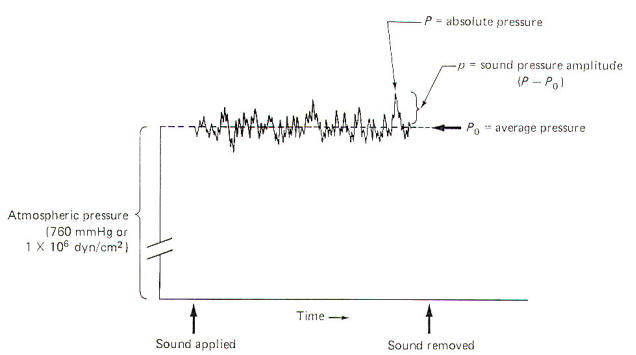
When no sound is disturbing the air, the average pressure Po at sea level is 1 atm. This is equivalent to 760 mmHg or 1 X 106 dyn/cm2. Sound pressure waves are superimposed on this average pressure. Since sound waves are oscillatory, the instantaneous absolute pressure P periodically varies above and below the average pressure. The sound pressure amplitude p ; which is utilized for calculations in sound and hearing studies, is equal to the difference between the average and absolute pressures (Fig-1). The sound pressure amplitude is usually expressed as dynes per square centimeter. The threshold of human hearing for a 1000-Hz pure tone is 2 x 10-4 dyn/cm2.
The sound pressure is usually expressed relative to the threshold of hearing. This relationship, called the sound pressure level, is measured in decibels (dB) and is calculated by the following equation: dB = 20 log Pi/Po where Pi = actual sound pressure amplitude Po = reference level sound pressure amplitude (typically the hearing threshold) dB = sound pressure level in decibels A factor of 10 change in the sound pressure amplitude represents a 20-dB change in the sound level. For example, a conversational level of sound is about 2 x 10-1 dyn/cm2 and is thereby 60 dB greater than the reference threshold level of 2 x 10-4 dyn/cm2. The discomfort level is about 2 x 102 dyn/cm2 (120 dB). We will deal with this equation again a little later on.
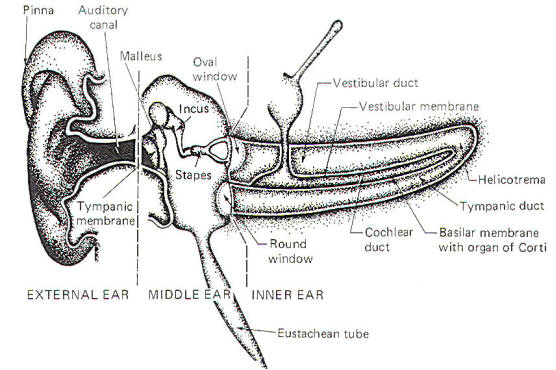
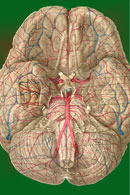
The ear is a mechanical transducer which converts the mechanical energy of oscillating air into impulses on the cochlear portion of the vestibulocochlear nerve (VIII). It is composed of an external, middle, and inner portion (Fig-2).
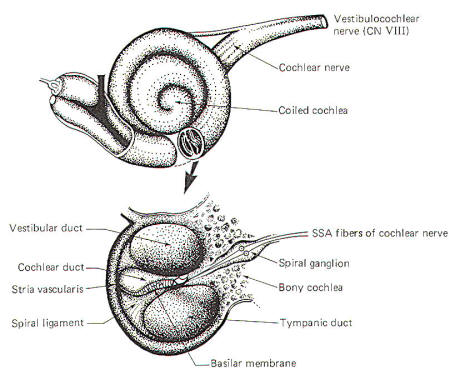
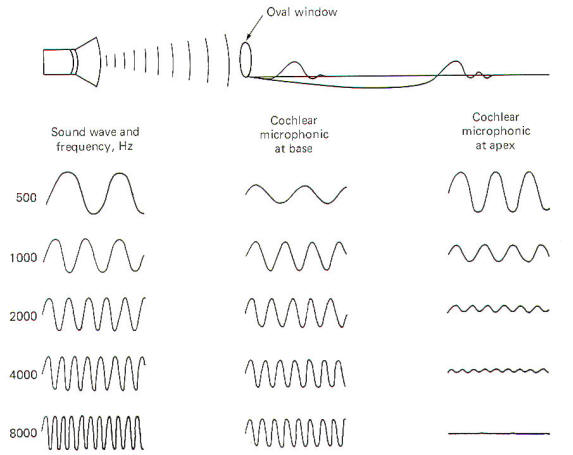
The external ear is composed of the part lying outside of the head (the pinna) and the auditory canal. The auditory canal ends at the tympanic membrane (eardrum), which separates the external from the middle ear. The middle ear is composed of the bony ossicles (malleus, incus, and stapes) along with their muscles and ligaments. It is separated from the inner ear by two thin, flexible membranes, the oval and round windows. The pressure in the middle ear is kept atmospheric by adjustments through the eustachian tube which opens into the nasopharynx. The inner ear is composed of the cochlea, a spiral fluid-filled tube approximately 3.5 cm long. Actually the cochlea is composed of three fluid-filled compartments. Two of them, the vestibular duct (scala vestibuli) and tympanic duct (scala tympani) are filled with perilymph, a fluid with many of the same ionic constituents as extracellular fluid. The fluid in the two ducts is continuous only at the helicotrema at the extreme apical end of the cochlea. The two ducts are separated from each other by a third cochlear duct (scala media) for most of the cochlear length (Figs-2 and 3). The cochlear duct contains a fluid called endolymph, which is similar to intracellular fluid in ionic concentration but is noticeably low in protein. It is separated from the vestibular duct by the vestibular (Reissner's) membrane and from the tympanic duct by the basilar membrane. Fixed on the basilar membrane is the mechanosensitive portion of the cochlea, the organ of Corti (Figs-3 and 4). Figure-3 illustrates the cochlea in its normal coiled form. The cochlea makes 2.5 turns in forming its coil. The bottom illustration in Fig-3 shows a cross section of the cochlea, clearly illustrating each of the three ducts. The basilar membrane separating the cochlear from the tympanic duct is narrow (0.04 mm) at its base near the oval window and becomes progressively wider (0.5 mm) at its apex near the helicotrema. The basilar membrane is attached to the outer wall of the cochlea by the fibers of the spiral ligament. Also on the outer wall of the cochlear duct is a secretory structure called the stria vascularis. Centrally the membrane is attached to a bony protuberance of the central pillar.
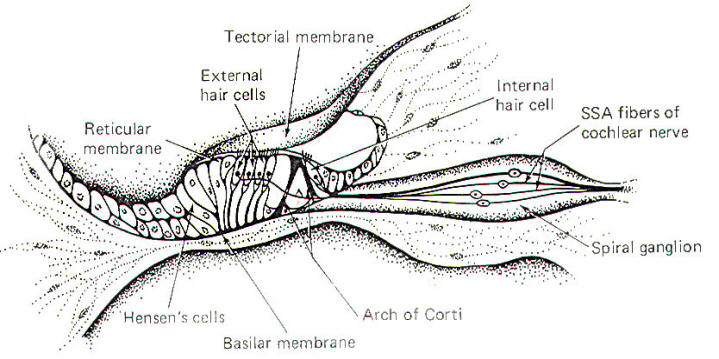
The organ of Corti, a sensitive structure resting on the basilar membrane, is responsible for converting mechanical oscillations of sound into impulses on the cochlear nerve. Because of its unique position, the organ of Corti is particularly sensitive to vibrations of the basilar membrane. A stiff but flexible arch of Corti supports a reticular membrane which is penetrated by hair cells. The internal and external spiral tunnels formed by this arch contain a fluid very much like perilymph (Fig-4).
A tectorial membrane makes contact with the individual hair processes of the hair cells. The inner hair cells have 40 to 60 hairs per cell, while the outer hair cells have as many as 80 to 100. While the hairs of the outer cells actually touch the tectorial membrane in the resting state, those of the inner cells apparently contact the membrane only during part of the oscillatory cycle of the basilar membrane. The peripheral endings of SSA fibers of the cochlear neurons make contact with both inner and outer hair cells. Typically one nerve fiber will innervate a single inner hair cell, whereas a single nerve fiber may innervate up to five or ten outer hair cells. The significance of this innervation pattern is not understood. As the stapes move in and out with the vibrations of middle ear, the oval window and perilymph of the vestibular duct are also set into oscillation. These vibrations are, in turn, transmitted to the endolymph of the cochlear duct through the vestibular membrane. Movement in the cochlear duct, in turn, sets the basilar membrane and organ of Corti into motion. The pressure waves are ultimately absorbed by the perilymph of the tympanic duct and damped at the round window.
Oscillations in the air are converted into oscillations in the ossicles and ultimately into oscillations of the fluids in the cochlea. The traveling waves which are set up on the basilar membrane near the oval window move outward over the membrane toward its apex near the helicotrema. Each area of the basilar membrane has a natural frequency or resonant point where it responds with maximum amplitude to the passage of the traveling wave. High-frequency sounds cause maximum oscillation of the membrane near the base and then quickly die out. Low-frequency sounds, on the other hand, cause the membrane to oscillate throughout its entire length but with the greatest amplitude near the apex (Fig-5). The natural frequency or resonant point of the basilar membrane decreases steadily from base to apex.
The organ of Corti hair cell is mechanosensitive. That is, it responds to the mechanical displacement of its hairs. A stimulated hair cell can initiate impulses in cochlear nerve fibers because the fibers end in tufts around the base of the hair cells. However, the mechanism by which an excited hair cell stimulates and produces impulses in the nerve fibers is still uncertain. To examine this process, let's begin by describing changes in the electrical activity of the cochlea in response to sound. The stria vascularis secretes K+ ions into the endolymph of the cochlear duct. This contributes to the establishment of an electrical potential across those membranes separating endolymph from perilymph. It's called the endocochlear potential and is typically about 80 mV with the endolymph positive relative to the perilymph. In addition, there is a potential difference across the membrane of the hair cell itself with the inside about 80 mV negative with respect to the outside. Thus there is a total potential difference of approximately 160 mV between the endolymph in contact with the hair cells and the cytoplasm of the cells. When the basilar membrane is stimulated by sound pressure waves propagated through the cochlear fluids, it alternately moves up and down in response to the frequency of the sound wave. Even though this displacement is small (about 3 µm at the resonant point), it is sufficient to excite the hair cells by altering the potential difference across their membranes. As the basilar membrane goes through its upward half-cycle, the reticular membrane with its hair cells moves upward and backward. Alternatively, as the membrane goes through its second half-cycle it moves downward and forward. Because the hairs are in contact with the tectorial membrane. they bend one way on the up cycle and the opposite way on the downward cycle. If a recording electrode is placed in the endolymph of the cochlear duct and a reference electrode placed in the perilymph, an oscillating potential called the cochlear microphonic potential (CMP) can be recorded when a sound is presented to the ear. This potential has both a positive and negative component. As might be expected, low-frequency sounds produce higher amplitude CMPs near the apex of the basilar membrane, while those produced by highfrequency sounds are larger near the base (Fig-5). If the cochlear microphonic potential plays any role in impulse production in the cochlear nerve fibers, it has not yet been established. The positive and negative components of the CMP vary with the upward and downward movements of the basilar membrane. As the membrane moves upward, the hair cell membranes are thought to depolarize and impulses are generated in the cochlear nerve fibers tufted around their bases. Alternately, as the basilar membrane moves downward the hair cell membranes hyperpolarize, decreasing impulse production in the nerve fibers. When no sound is presented to the ear, the basilar membrane is quiet. Nevertheless, there is a small but basal firing rate of about 50 impulses per second on the nerve fibers which alternately increases and decreases during oscillations of the membrane. A single cochlear nerve fiber has a maximum firing rate of about 1000 impulses per second
A sound wave approaching the ear must displace the tympanic membrane, the ossicles, and the fluid of the vestibular duct before it can displace the basilar membrane and organ of Corti, generating impulses in the cochlear nerve. During this transfer from an air pressure wave to a fluid pressure wave, the sound loses about 40 dB at the oval window. This is due to the fact that the vestibular perilymph has greater inertia than the air. To compensate for this loss of intensity, the auditory canal and the ossicular system ordinarily amplify the incoming sound pressure wave by about 35 dB. Because of this amplification, little or no loss in sound intensity occurs as the wave is transferred from the air to the fluid medium. This is an example of impedance matching. In other words, the loss in intensity due to the fluid inertia is compensated for by an equally strong previous amplification.
As an example, assume that a 1000-Hz pure tone with a sound pressure amplitude of 2 x 10-1 dyn/cm2 is presented to the ear. A sound with these characteristics will be amplified about 10 dB as it travels through the auditory canal of the outer ear and another 25 dB as it travels through the ossicular system of the middle ear. The 10-dB gain through the auditory canal can be explained because it behaves exactly like sound in a closed tube. The sound pressure is about 3 times greater at the closed end than at the open end. This translates to a 10-dB gain as follows: dB = 20 log (sound pressure at eardrum/sound pressure entering canal) = 20 log (6 x 10-1 dyn/cm2 / 2 x 10-1 dyn/cm2) = 10-dB gain through the auditory canal A more complex problem is explaining the 25-dB gain through the ossicular system as several factors are involved. Three initial physical characteristics of the ear are required: the surface areas of the tympanic membrane, that portion of the membrane in contact with the manubrium, and faceplate of the stapes in contact with the oval window. 0.66 cm2 = surface area of tympanic membrane 0.55 cm2 = surface area of membrane in contact with manubrium 0.032 cm2 = surface area of faceplate of stapes Because of the threefold increase in amplitude gained through the auditory canal, the sound pressure on the tympanum is 6 x 10-1 dyn/cm2. Since only 0.55 cm2 of the membrane is actually in contact with the manubrium of the malleus, the force produced on the malleus can be calculated as follows: Force (on malleus) = area (of malleus) x pressure (on tympanum) = (0.55 cm2) (6 x 10-1 dyn/cm2) = 3.3 x 10-1 dyn Experimental models indicate that the vesicles have a theoretical mechanical advantage of 1.3. Therefore, the force on the stapes can be calculated as follows: Force (on stapes) = 1.3 x force (on malleus) = 1.3 (3.3 x 10-1 dyn) =4.29 x 10-1 dyn Given that the area of the faceplate of the stapes is 0.032 cm2 and knowing the force on the stapes, the pressure on the oval window can be calculated as follows: Sound pressure (on oval window)=force (on stapes)/area (of stapes) = 4.29 x 10-1 dyn/0.032 cm2 = 1.34 x 101 dyn/cm2 Using the pressure on the tympanum as the reference level, the gain through the ossicular system can now be calculated. dB = 20 log pressure (on oval window) /pressure (on tympanum) =20 log (1.34 x 101 dyn/cm2/6 x 10-1 dyn/cm2) = 27-dB gain through the ossicles The 27-dB gain calculated above ignores friction and damping, however. Thus the actual recorded experimental value is closer to 25 dB. Consequently the total gain through the outer and middle ear is approximately 35 dB. This is certainly important as the air-fluid interface at the oval window reflects about 99 percent of the sound energy back to the air. This represents a 40-dB loss in transmission and is calculated as follows: dB = 20 log (pressure (in perilymph)/ pressure (on oval window)) = 20 log (1.34 x 10-3 dyn/cm2/1.34 x 10-1 dyn/cm2) = -40-dB loss at oval window It is important to realize that impedance matching is never perfect. It is probably 50 to 90 percent perfect for sound waves in the 300- to 3000-Hz range. This allows almost full utilization of the energy in the incoming sound wave. However, at very high and very low frequencies, the impedance becomes higher and thus the impedance matching becomes poorer. Consequently a higher threshold for hearing is observed in these ranges. The natural resonating frequency of the ossicular system is between 700 and 1400 Hz. However, due to the action of ligaments and muscles in the middle ear, the system is slightly damped, causing sound waves of 1200 Hz to be transmitted through the ossicular system with slightly greater ease than sound waves of any other frequency. The natural resonating frequency of the auditory canal is about 4000 Hz and thus selectively favors waves of this frequency. Therefore, combining the resonating effects of the auditory canal and the ossicular system, the best transmission of sound waves from air to the inner ear is for sound waves in the 1200to 4000-Hz range. Transmission is not as good above and below this range.
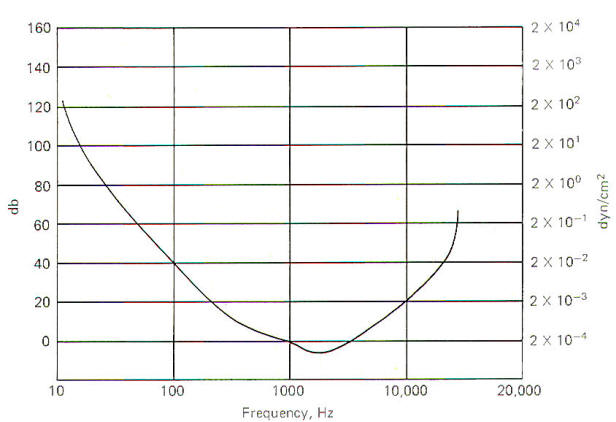
The threshold of hearing is a function of sound frequency and intensity (Fig-6). Under ideal laboratory conditions, the threshold of hearing for a 1000Hz pure tone is 2 x 10-4 dyn/cm2. However, as the sound frequency decreases, the threshold for hearing increases. For example, the sound intensity would have to be 2 x 10-2 dyn/cm2 in order to just be able to hear a 100-Hz pure tone. This is 40 dB greater than would be required to just hear a 1000-Hz pure tone. Notice that the most sensitive range is between 1200 and 4000 Hz. Points on the curve represent the threshold of hearing for each combination of frequency and intensity. A threshold point is established when the subject hears the tone 50 percent of the time it is presented. A 130-dB sound is felt as well as heard and eardrum rupture is a real possibility at 160 dB.
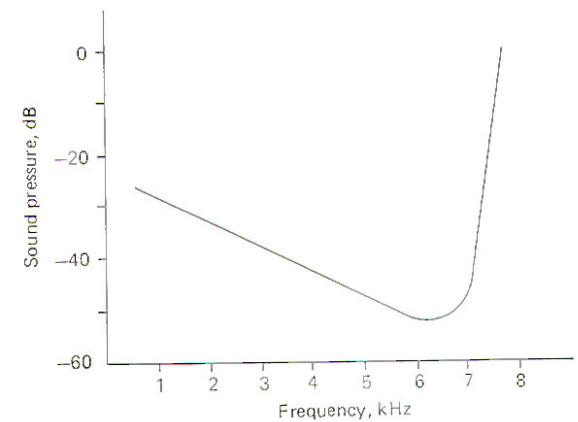
When sounds of different frequencies are presented to the ear and responses are recorded from a single cochlear nerve fiber, it can be seen that the fiber has a characteristic or "best" frequency. This is the frequency to which the fiber responds with the least intensity (Fig-7). Notice that as the frequency is increased or decreased from the best frequency the intensity of the sound required to fire the fiber increases. The best frequency of the nerve fiber illustrated is about 6.5 kHz. Thus while it is apparently true that each area of the basilar membrane responds maximally to a relatively narrow band of frequencies, it will respond, although with less sensitivity, to a broader range as well.
We ordinarily pay very little attention to the background noise in our immediate environment. However, if we wish to single out a particular sound from all others we can often do it by the conscious effort of directing our attention to that sound to the exclusion of all others. While the mechanisms by which this is done are not known, one intriguing possibility is based on the discovery of auditory efferents. While their origin is largely unknown, the possibility exists that they may function by inhibiting the basilar membrane to tones on either side of the desired frequency. This would in effect "sharpen" or add "contrast" to the desired frequency range.
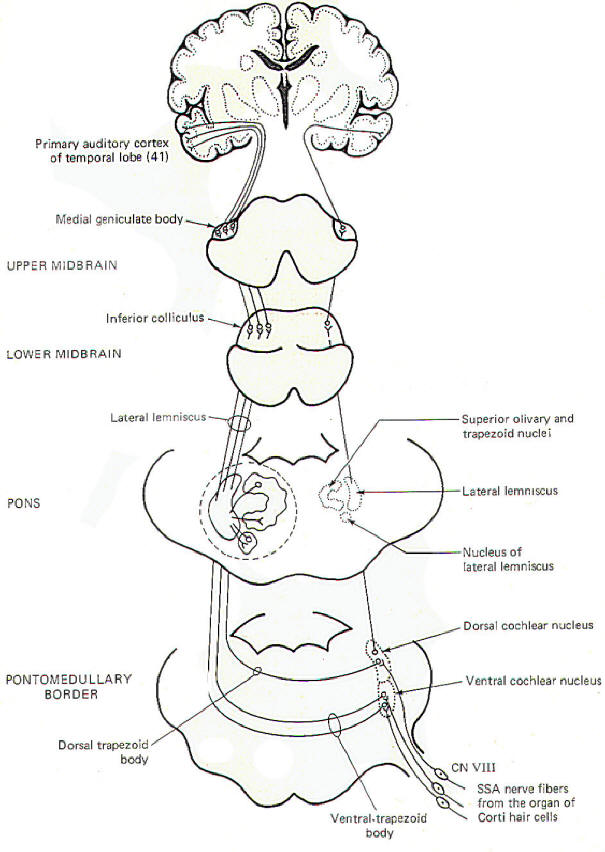
In addition to the conscious awareness of sound, which is mediated over the conscious pathways just described, humans are also subject to a variety of auditory reflexes. A sudden, loud, unexpected sound can cause reflex quickening of the pulse, increased blood pressure, and sudden movements of the eyes, head, neck. and the whole body. Cardiovascular and other visceral responses are mediated over the autonomic nervous system. While the exact relationship between the auditory system and the autonomic nervous system is not known, the second-order neurons from the cochlear nuclei do send some collateral fibers to the brainstem reticular formation in addition to the other routes previously described. The relationship between the reticular formation and the autonomic nervous system is well documented and probably plays some role in the cardiovascular and visceral reflexes in response to sound. Reflex eye movements are mediated by input from the cochlear nuclei to both ipsilateral and contralateral motor nuclei of cranial nerves III, IV, and VI via the medial longitudinal fasciculus. Reflex movements of the head, neck, and body in response to sound are probably mediated over reflex pathways from the cochlear nuclei to the midbrain tegmental nuclei. Descending signals over the tectospinal tracts from these nuclei then produce the appropriate movements.
If the head of a person with normal hearing is fixed in a restrainer so that it can't be moved in any direction, that person will probably have little difficulty in determining the direction from which a sound is coming. However, a person totally deaf in one ear will have great difficulty in localizing the sound. The implication is that input to both ears (binaural input) is necessary for sound localization. Similarly, a person with damage to the auditory cortex will also have difficulty with sound localization. Thus it appears that central interaction of the auditory input is also a necessary component of the process.
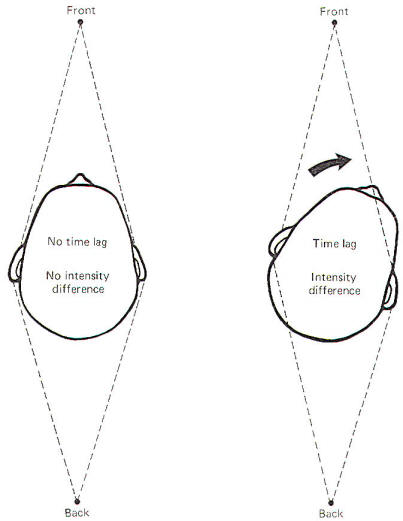
Figure-9 illustrates how the location of a sound with respect to the head causes a time lag and intensity difference between both ears. When a sound source is directly in front of the head, the sound reaches each ear at exactly the same time (no time lag) and with the same intensity (no intensity difference). Of course the same is true if the sound source is directly behind the head. Moving the head slightly to the left or right creates a time lag and intensity difference which provide clues needed for sound localization. For example, if the sound source is directly in front and the head is turned to the right, the sound reaches the left ear earlier and with greater intensity than it does the right. The intensity difference is caused primarily by the fact that the head itself serves as somewhat of a sound shield. Of course if the sound source were directly behind the head, moving the head to the right would produce just the opposite effects in time lag and intensity difference. Thus it is important to recognize that sound localization is aided by moving the head, a response most of us perform automatically when localizing sounds without even thinking. Obviously, sound localization when the head is fixed in space is much more difficult.
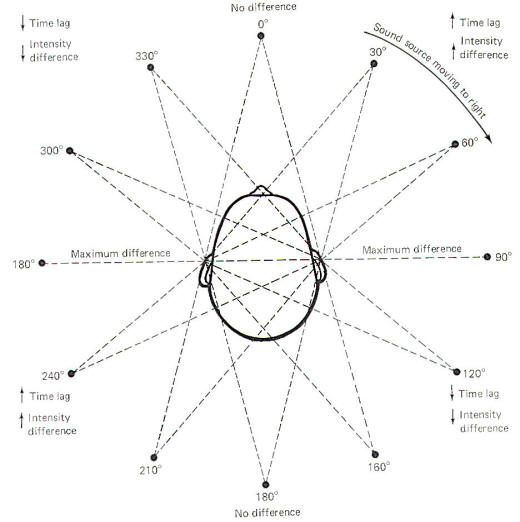
If a sound source starts directly in front of the head and then moves to the right in a circle around the head, time lag and intensity differences will constantly be changing (Fig-10). As it moves through the first 90° quadrant, the time lag and intensity difference increase to a maximum. As the sound source continues to move around the fixed head, through the remaining 270° of the circle, predictable changes in time lag and intensity difference occur. Moving the head is necessary for quadrant localization. Notice that the time lag and intensity difference are essentially the same when the sound source is at the 30 and 160° positions. Consequently these two clues are not enough to tell the brain from which direction the sound is coming. The additional clue necessary for localization is provided by moving the head. When the head is turned slightly to the right, there will be a decrease in the time lag and intensity difference if the sound source is at the 30° position, and an increase in both of these parameters if the source is in the 160° position.
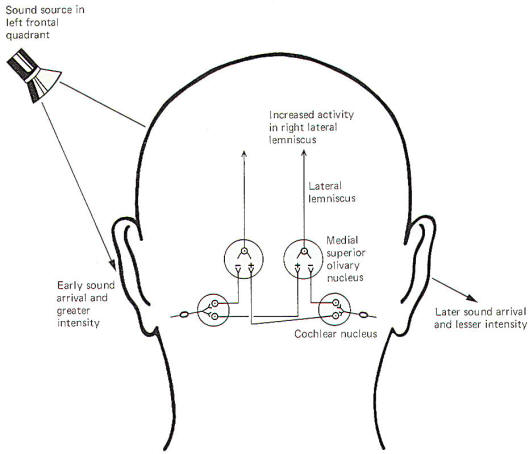
The central neural mechanisms for detecting sound direction are unknown. The following is a hypothesis based on observed available evidence. When recording electrodes are placed on certain projection neurons of the medial superior olivary nucleus of the cat, a lateral source of sound causes a net increase in activity in the contralateral nucleus and a net decrease in the ipsilateral nucleus. In other words, if the sound source were in the left frontal quadrant of the cat, the right lateral lemniscal fibers from the medial superior olivary nucleus to the inferior colliculus would show a relative increase in firing rate, while the left lemniscal pathway would show a decrease. This net "weighting" of activity toward the contralateral side provides the signal for sound lateralization.
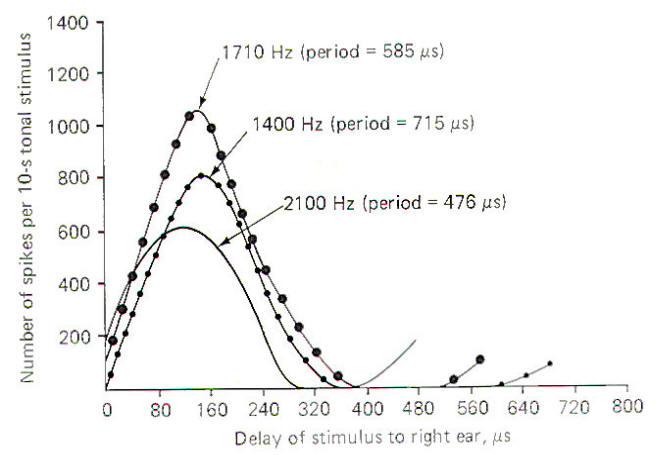
Figure-12 illustrates the response of a single collicular neuron to tones of three different frequencies in the cat when the sound source arriving at the right ear is increasingly delayed with respect to the left ear. Notice that as the sound source moves from a position directly in front of the head (zero delay) progressively to the left, the delay of stimulus to the right ear produces a characteristic pattern of impulse firing in the collicular neuron. The firing rate increases to a maximum at about 140 µs and then decreases again. The delay time which produces the greatest firing rate is the characteristic delay of that neuron. Thus the characteristic delay in this case is 140 µs. Notice that the actual value of the maximum firing rate varies with the frequency of the tone, but the characteristic delay remains the same. Since different sound-delay neurons respond maximally to different time lags, the brain interprets the location of a sound by a combination of which sound location neurons are maximally firing. An assumption is made that the maximum signal a neuron can produce is an important message for the brain with the sound-delay neurons providing the signal for localization.
It is likely that the right auditory cortex recognizes that when certain sound localization neurons fire at their maximum rate, the sound is at a particular location in the left auditory field. A possible mechanism for sound localization is the following. Assume that a sound is located at a particular point in the left auditory field so that the sound delay is 120 µs. Those contralateral inferior collicular neurons with characteristic delays of 120 µs will respond with their maximum firing rate. Presumably the right auditory cortex "recognizes" that when this particular group of neurons is firing maximally, the sound is at a particular location in the left auditory field. Now if the sound were to move even further to the left, the sound delay would increase and a group of neurons with characteristic delays equal to this new time lag would begin to fire maximally This would signal the new position of the sound to the auditory cortex. The left auditory cortex is no doubt also involved in this process. Sound lateralization (determining whether the sound is coming from the left or the right) is probably based on a different mechanism. The relative weighting of activity to the contralateral lateral lemniscus, and thus ultimately to the contralateral auditory cortex, may be the clue for lateralization. If the brain detects a net increase in firing in the left lateral lemniscus, it "knows" that the sound is coming from the right auditory field, and vice versa.
|
|
|
Copyright [2007] [CNS Clinic-Jordan]. All rights reserved