|
|
|
 |
|
|
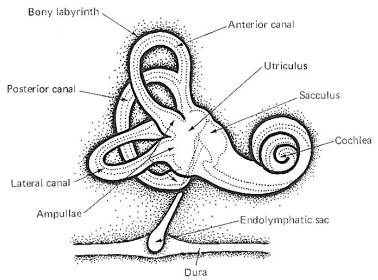
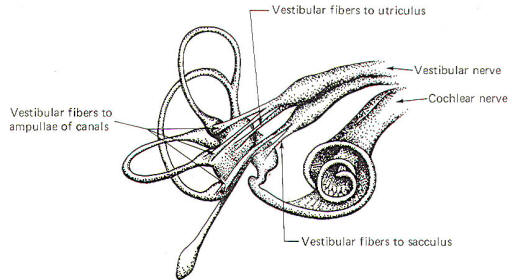
The vestibulocochlear nerve (VIII) has the dual function of serving both the sense of hearing (via cochlear fibers) and proprioception (via vestibular fibers).
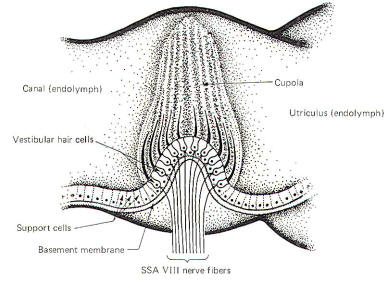
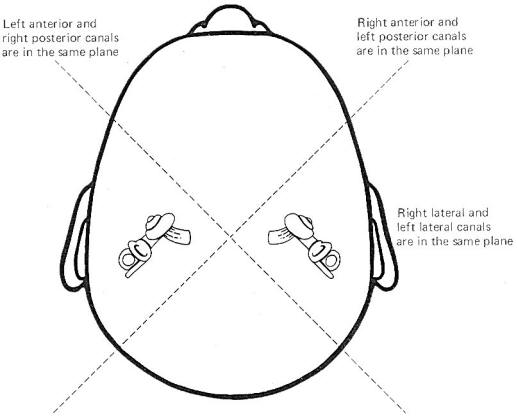
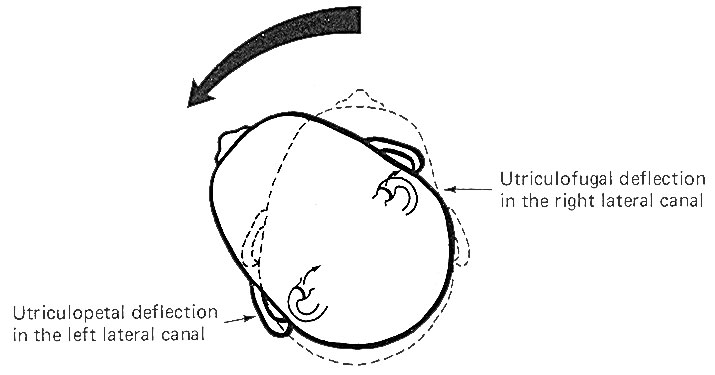
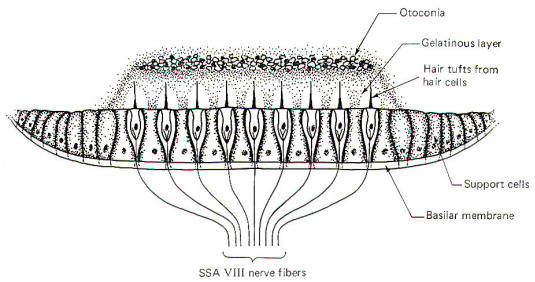
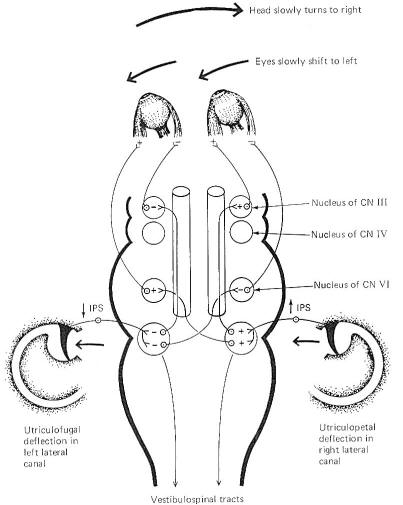
The hair cells in a given crista ampullaris are all orientated in the same direction so that deflection of the cupola either bends all the hairs toward the kinocilia or away from it. Thus deflection of the cupola either increases or decreases the firing rate of the SSA VIII nerve fibers. In the lateral canals, the kinocilia all face the utriculus. In the vertical canals they all face away from the utriculus, toward the canal. Thus, utriculopetal deflection in the lateral canals produces an increase in the firing rate, while utriculofugal deflection produces a decrease. However, just the opposite is true concerning the vertical canals. Here the hair cell kinocilia are oriented in the opposite direction so that utriculopetal deflection causes a decrease while utriculofugal deflection produces an increase in the firing rate. Coplanar Canals are Functional Units The anterior canal on one side of the head and the posterior canal on the opposite side are in the same plane. Thus the two canals are a functional unit since any head movement which causes utriculofugal deflection in the anterior canal on one side will be matched by utriculopetal deflection in the posterior canal on the opposite side (Fig-6). A similar relationship exists with the two lateral canals and they also form a functional unit (Fig-7).
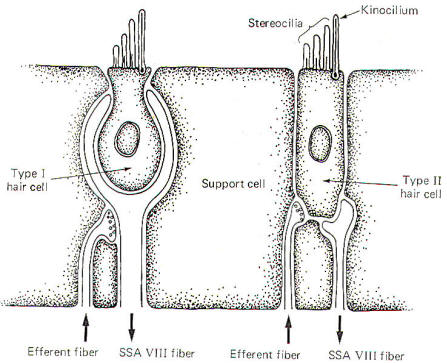
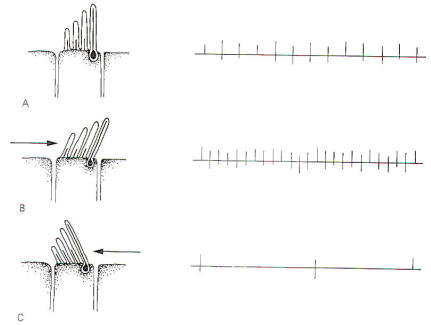
Hair cells stimulate SSA VIII nerve fibers via chemical synapses. Because a fairly steady resting discharge of 40 to 60 impulses per second can be recorded in the nerve fibers, it is assumed that a small amount of transmitter chemical (possibly a catecholamine) is constantly being released. It has been proposed that displacement of the hairs toward the kinocilium increases the firing rate by increasing the rate or amount of transmitter released by the hair cell. Likewise, displacement of the hairs in the opposite direction decreases the firing rate by lowering the rate or amount of release. In contrast to the stereocilia, which are embedded in the cuticle, the base of the kinocilium is in direct contact with the hair cell cytoplasm. The kinocilium plunging inward (with the aid of the stereocilia leaning against it) may depolarize the hair cell membrane and establish a receptor potential, which in turn causes transmitter release. Alternatively, deflection of the stereocilia away from the kinocilium pulls the kinocilium outward, hyperpolarizing the membrane and decreasing transmitter release. The cristae are particularly sensitive to changes in angular acceleration and deceleration of the head. The greatest change in firing rate along nerve fibers from the cristae occur at the beginning and end of angular movements of the head. As Fig-7 shows, the inertia of the endolymph when the head first starts rotating to the left produces utriculopetal deflection in the left canal and utriculofugal deflection in the right canal. Thus we see a large initial change in firing rate from each canal at the beginning of the movement. However, if the rotation of the head to the left continues, we see no further change in firing rates until the rotation begins to slow down (decelerate). At this point. the inertia of the endolymph causes the cupola to deflect in the opposite direction, once again causing a change in the firing rate. This time, however, there is a decrease in the left canal and an increase in the right canal. Thus one can see that the canal system is particularly adept at signaling changes in acceleration and deceleration of the head's angular movements. Further, because the canals are arranged in three planes, angular movements in all directions are easily detected by the canal system. No doubt angular movements which are not exactly parallel with a single coplanar canal system are detected by the brain through some "weighted" input from two or more coplanar functional units.
Because of the role the vestibular system plays in the maintenance of posture and muscle control, it is not surprising to find that the system has a close relationship with the cerebellum. Both first- and second-order vestibulocerebellar fibers end as mossy fibers on the granular cells of the cerebellar cortex of the flocculonodular lobe. In addition, the fastigial and dentate cerebellar nuclei also receive vestibular input. Presumably the cerebellar cortex integrates the vestibular input with other proprioceptive input from all parts of the body. The cerebellum is then in a position to exert influence on the postural musculature via output to the vestibular, reticular, and red nuclei. Vestibulospinal, reticulospinal, and rubrospinal fibers influence muscle activity at the spinal cord level, while cerebellar output through the thalamus to the cerebral cortex modifies motor activity at the cortical source.
In order to be consciously aware of position and movements of the head in space, it is necessary that vestibular information reach the cerebral cortex. The kinesthetic sense (conscious awareness of body position and movement) requires cortical input from peripheral proprioceptors as well as from the vestibular system. The cortical area which receives this information is located in the postcentral gyrus near the somatosensory projection of the mouth. Vestibulocortical projections appear to be primarily contralateral with intermediate synapses in the ipsilateral vestibular nuclei and the contralateral thalamus.
The effects of vestibular activity on autonomic function are well known and are grouped under the heading "motion sickness." They include effects on the vasomotor system (typically a vasodepressor action with a blood pressure drop), an increase in the rate and depth of respiration, decreased salivation, increased sweating, pupillary dilation, and disturbances of the gastrointestinal tract. Most of these effects are mediated through the sympathetic nervous system.
Certain bodily responses to vestibular stimulation are reflexly predictable, such as conjugate movements of the eyes and other postural adjustments of the body. The integrity of the various canals can be tested by their capacity to produce the expected responses. The rotation (swivel chair) test and the caloric test are both designed to do this. The rotation test allows maximum stimulation of the horizontal and vertical canals. Maximum deflection of the cupola of a particular canal occurs when the movement of the head is in the same plane as the canal which contains that cupola. This is accomplished in the swivel chair by placing the head in various positions and then rotating the chair. Recall that maximum deflection in a canal on one side of the head is accompanied by maximum deflection in its functional counterpart on the opposite side. Predictable responses observed with rotation tests are nystagmus, vertigo, and past pointing. Nystagmus refers to rapid to-and-fro movements of the eyes. As previously noted, the eyes slowly shift to the left as the head is turned slowly to the right. Of course there is a limit to how far left the eyes can shift if the head continues turning to the right. When they have pulled as far left as possible, they suddenly "snap" back to the right and "fix" on a new reference point in the visual field. This alternating slow phase to the left followed by a fast phase to the right continues as the head keeps rotating to the right unless consciously overridden. While nystagmus technically refers to the eye shifts in both directions, neuroscientists typically refer to nystagmus as the direction of the fast phase. For example, nystagmus is to the right in the case just described. Because cupola deflection directly controls eye movements, and because this deflection is in one direction during the acceleration phase of the angular rotation and in the opposite direction during the deceleration phase, it follows that nystagmus is in one direction during rotation (perrotation) and in the opposite direction after rotation (postrotation). Perrotational nystagmus is in the same direction as the rotation. However, if the rotating chair is suddenly stopped, the canals cease to rotate but the inertia of the endolymph is not so easily overcome. Consequently the cupolae are deflected in the opposite direction for a brief period of time, producing a postrotational nystagmus in the direction opposite the rotation. Vertigo and past pointing are also predictably observed following rotation in a normal individual. Vertigo is the sensation of a movement when no such movement exists. This is caused by the fact that once the actual turning stops, the inertia of the endolymph remains for a while, deflecting the cupolae and sending signals to the brain that turning is still occurring. Normally the vertigo (false sense of movement) is in the same direction as the postrotational nystagmus. The body will ordinarily attempt to reflexly make postural adjustments for the vertigo just as it would for a real movement. Thus, predictable leaning of the whole body (a reflex attempt to correct for the false movement) is typically observed following a period of rotation. Specifically, the body leans in the direction opposite the postrotational nystagmus, An extended arm also points in the direction opposite the post rotational nystagmus. This is past pointing, The rotation test has the disadvantage of not allowing the canals on each side of the head to be tested separately. However, caloric tests, which involve the introduction of hot or cold solutions into the auditory canal, allow the clinician to test each side of the head separately. A hot water solution introduced into the auditory canal causes the endolymph to expand, deflecting the cupola in a predictable direction. This is later followed by the use of a cold water solution which cools the endolymph, producing deflection in the opposite direction. Like the rotation test, predictable changes in nystagmus, vertigo, and past pointing can be observed.
|
|
|
Copyright [2007] [CNS Clinic-Jordan]. All rights reserved